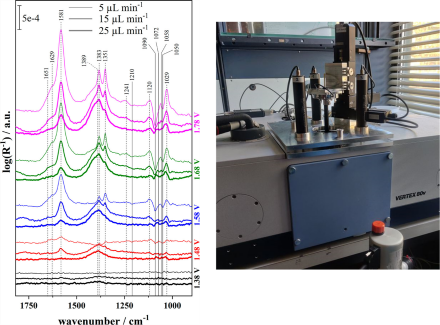
In-situ ATR-FTIR spectroscopy is a powerful analytical technique for detecting a huge variety of different compounds. Our group has been closely working together with the industrial chemistry department of Prof. Muhler in using this technique to study various electrocatalytic alcohol oxidation reactions. Using a flow-through borehole electrode, we can couple ATR-FTIR and electrochemistry to monitor species involved in the reaction such as the reactant, products, or the intermediates in the thin electrolyte film that is formed between the internal reflection element (IRE) and the catalyst surface. Thus, we can study the effect of key electrolysis parameters such as distance between IRE and electrode, electrolyte flow rate and applied potential
S. Cychy, D. Hiltrop, C. Andronescu, M. Muhler, W. Schuhmann, Analytical chemistry 2019, 91, 14323–14331..
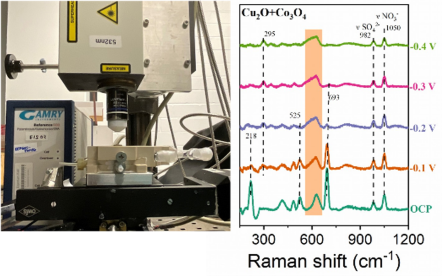
In-situ Raman spectroscopy is a widely used analytical technique which can provide more sensitive real-time insights into the structure and phase evolution process under operating conditions. Thus, when combined with our self-made Raman electrochemical cell, we can gain valuable information on what happens to the catalyst during complex reactions such as CO2 reduction reaction and NO3- reduction reaction.
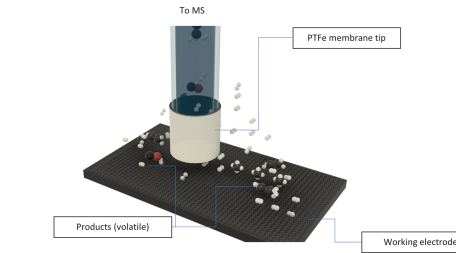
Differential electrochemical mass spectrometry (DEMS) is a powerful technique that can be used to identify and quantify gaseous products or intermediates of continuous faradaic reactions. We use different static as well as flow cells which are connected to a conventional quadrupole mass spectrometer via a hydrophobic porous membrane to separates the gaseous molecules from water. Various electrochemical methods (Voltammetry, Galvanometry, RDE) can be applied. Systems under study are e.g. CO2 reduction, carbon corrosion