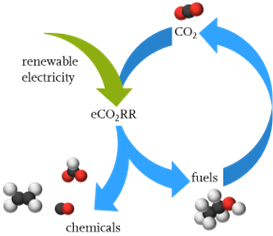
The electrochemical CO2 reduction reaction (eCO2RR) is a key technology for converting a major driver of climate change into commercially valuable products, such as CO, HCO2-, C2H4 and C2H5OH, alongside reducing CO2 emissions. When the eCO2RR is powered by renewable energy, a closed carbon cycle economy can be envisaged. The incorporation of metal-based catalysts in gas diffusion electrodes will further allow the conversion of CO2 at high current densities, thus enabling industrial relevance.
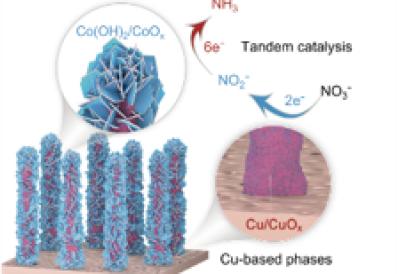
Ammonia (NH3) is an essential raw material in fertilizers, the chemical industry, and emerging energy conversion processes. Presently, the production of NH3 relies on the energy-intensive Haber-Bosch process, which is a gas-phase reaction between H2 and N2 under high temperature and pressure. The nitrate (NO3−) reduction reaction (NO3RR) stands out as a promising route due to the relatively lower energy and allow much faster reaction kinetics for NH3 production. Simultaneously, NO3− is widely available in industrial wastewater and groundwater, and hence the NO3RR can also address environmental pollution problems.
Searching for highly active, stable, and selective electrocatalysts for the oxygen evolution reaction (OER), oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER), which can be applied to water splitting, fuel cells and metal–air batteries, is critical for developing clean and renewable energies. Various catalyst materials as well as bi-functional catalysts for water electrolysis or OER/ORR are investigated. Measurements are performed using rotating disk electrode (RDE) or rotating-ring disk electrode (RRDE) techniques, as well as in small model flow-through electrolyzers with electrolyte-filled gap or zero-gap membrane electrode assembly configuration.
Searching for highly active, stable, and selective electrocatalysts for the oxygen evolution reaction (OER), oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER), which can be applied to water splitting, fuel cells and metal–air batteries, is critical for developing clean and renewable energies. Various catalyst materials as well as bi-functional catalysts for water electrolysis or OER/ORR are investigated. Measurements are performed using rotating disk electrode (RDE) or rotating-ring disk electrode (RRDE) techniques, as well as in small model flow-through electrolyzers with electrolyte-filled gap or zero-gap membrane electrode assembly configuration.
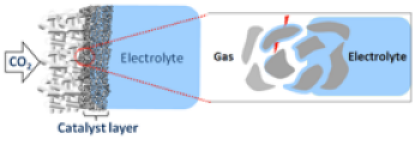
Electrocatalytic conversions of gaseous reactants such as CO2 at industrially relevant conditions require a fast mass transport of gas molecules to liquid/solid electrochemical interfaces. While the gas transport via the bulk electrolyte phase imposes severe diffusional restrictions, this problem is mitigated by gas-diffusion electrodes, where the bulk liquid and gas phases are separated by a porous electrode structure. Our group does not only develop spray-coating protocols for the home-made production of gas-diffusion electrodes, but also studies the influence of pore structure and hydrophobicity on the performance of the CO2 reduction reaction.
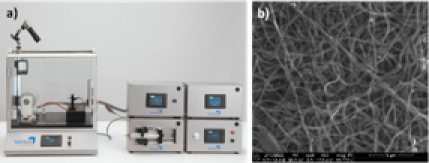
A spray-coating platform is employed for the development of multi-metal catalysts via the aerosol-synthesis method or for creating polymer/metal precursor composites, where metal precursor particles are entrapped in the foamy polymer matrix and can be further converted to catalyst materials by thermal processing. Using an electrospinning platform (Figure 1a) it is possible to create catalyst fibers in order of some hundred nanometers (Figure 1b). Catalyst materials are investigated using various techniques like scanning electron microscopy (SEM), transmission electron microscopy (TEM), inductively coupled plasma mass spectroscopy (ICP-MS), X-ray diffraction analysis (XRD), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy.
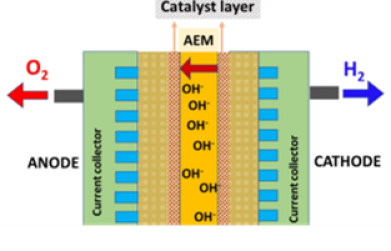
Water electrolysis is a key process for hydrogen production using renewable resources which can replace fossil fuel based nonrenewable energy production.
Our research interests