Climate change and its impact on life on our planet can only be mitigated if humankind manages to restrict further massive pollution, especially by emission of CO2 and NOx. In order to contribute to technological solutions, we develop knowledge and new experimental devices to in-depth investigate electrocatalyst-modified gas-diffusion electrodes for CO2 reduction. On the one hand, we develop new catalyst materials to convert CO2 into value-added chemicals such as ethylene or higher alcohols. On the other hand, we are convinced that an in-depth understanding of the complex set of parameters is of utmost importance to allow optimizing devices for CO2 reduction.
The overall objectives of CASCAT are to gain a deep understanding of electrocatalysis for CO2 reduction using gas diffusion electrodes. This is achieved by searching and characterizing new catalyst materials, developing new technologies for the fabrication of gas diffusion electrodes by airbrush-type spray coating, assembling the catalyst material(s) at the optimal location inside the gas diffusion electrode, and making use of (nano)electrochemical techniques, spectroelectrochemical techniques to elucidate properties of the working gas diffusion electrode.
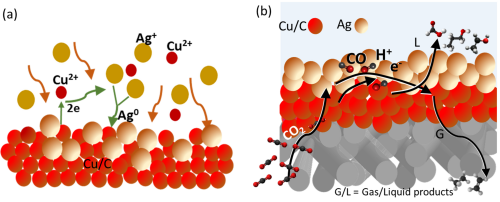
We designed Ag core/porous Cu shell catalyst particles and could demonstrate synergistic cascade reactions during CO2 reduction with the enhanced formation of ethylene, ethanol and propanol. By careful design of gas-diffusion electrodes, we found measures to mitigate the parasitic hydrogen evolution reaction by managing the electrolyte wetting inside the electrode by adjusting the hydrophobicity and porosity. We developed automatic measurement setups with in-line product analysis, nanoelectrochemical methods for characterizing single electrocatalyst nanoparticles, and in-situ electrochemistry/Raman spectroscopy methods to identify intermediate products during CO2 reduction. Most importantly, we developed and applied a nanoelectrode-based method which allows us to measure the local pH value in the gas diffusion electrode in dependence from the applied current density. First results concerning the implementation into model flow-through electrolyzer systems were obtained. Furthermore, we worked on the reduction of NOx and found that Cu/Co-based catalyst work synergistically for the reduction of nitrate to ammonia with nearly 95% yield.
Until the end of the project, we expect to develop improved electrocatalysts for CO2- and NOx-reduction and combinations of catalysts as a basis for cascade reactions enabling high selectivity for value-added chemicals and suppressing the parasitic hydrogen evolution reaction. We want to characterize the catalysts, the mechanistic details of the complex reaction pathways while keeping scalable synthesis, operational stability at high currents and abundance of the constituting elements in focus to potentially enable industrial applications.
I. A. Cechanaviciute, W. Schuhmann, ChemSusChem 18 (2025) e202402516. Electrocatalytic ammonia oxidation reactions: Selective formation of nitrite and nitrate as value-added products
J. Zhang, B. Kumari, T. Quast, S. Cychy, I. Sanjuán, M. Muhler, W. Schuhmann, C. Andronescu, Adv. Func. Mater. 35 2025) 2419911. Understanding how surface reconstruction induced by preconditioning in different electrolytes impacts electrooxidation of solketal on multi-metal-based catalysts 10.1002/adfm.202419911
G. Lu, X. Wang, J. Timoshenko, B. Roldan Cuenya, G. Zhao, X. Huang, W. Schuhmann, M. Muhler, Adv. Func. Mater. 35 (2025) 2419075. A 3D macroporous carbon NiCu single-atom catalyst for high current density CO2 electroreduction 0.1002/adfm.202419075
P. Wang, X. Wang, S. Chandra, A. Lielpetere, T. Quast, F. Conzuelo, W. Schuhmann, Angew. Chem. Int. Ed. 64 (2025) e202422882. Hybrid enzyme-electrocatalyst cascade modified gas-diffusion electrodes for methanol formation from carbon dioxide 10.1002/anie.202422882
J. Weidner, C. N. Tchassem, D. Das, R. Zerdoumi, G. Lu, X. Wang, M. Muhler, N. Sikdar, W. Schuhmann, ChemElectroChem 12 (2025) e202400664. Al-rich Cu/CuOx catalyst in a CO2-reduction tandem electrolyzer with CO-enriched gas feed for enhanced C2+-products selectivity 10.1002/celc.202400664
N. Jiyane, C. Santana Santos, I. Echevarria Poza, M. Palacios Corella, M. A. A. Mahbub, G. Marin-Tajadura, T. Quast, M. Ibáñez, E. Ventosa, W. Schuhmann, Battery Supercaps 8 (2025) e202400743. Recessed microelectrodes as a platform to investigate the intrinsic redox process of Prussian Blue analogs for energy storage application 10.1002/batt.202400743
M. A. A. Mahbub, D. Das, X. Wang, G. Lu, M. Muhler, W. Schuhmann, Angew. Chem. Int. Ed. 64 (2025) e202419775. Towards the use of low-concentration CO2 sources by direct selective electrocatalytic reduction. Angew. Chem. 137 (2025) e202419775. Auf dem Weg zur Nutzung von CO2-Quellen mit niedriger Konzentration durch direkte selektive elektrokatalytische Reduktion 10.1002/anie.202419775
R. P. Antony, L. Li, C. Santana Santos, N. Limani, S. Dieckhöfer, T. Quast, J. Weidner, W. Schuhmann, ACS Mater. Lett. 6 (2024) 5333-5339. Insights of the proton transport efficiency of a membrane electrode assembly by operando monitoring of the local proton concentration during water oxidation 10.1021/acsmaterialslett.4c01655
J. Zhang, T. Quast, B. Eid, Y.-T. Chen, R. Zerdoumi, S. Dieckhöfer, J. R. C. Junqueira, S. Seisel, W. Schuhmann, Nature Commun. 15 (2024) 8583. In-situ electrochemical reconstruction and modulation of adsorbed hydrogen coverage in cobalt/ruthenium-based catalyst boost electroreduction of nitrate to ammonia 10.1038/s41467-024-52780-x
G. Arruda de Oliveira, M. Kim, C. Santana Santos, N. Limani, T. D. Chung, E. Batsa Tetteh, W. Schuhmann, Chem. Sci. 15 (2024) 16331-16337. Controlling surface wetting in high-alkaline electrolytes for single facet Pt oxygen evolution electrocatalytic activity mapping by scanning electrochemical cell microscopy 10.1039/d4sc04407j
I. A. Cechanaviciute, B. Kumari, L. M. Alfes, C. Andronescu, W. Schuhmann, Angew. Chem. Int. Ed. 63 (2024) e202404348. Gas diffusion electrodes for electrocatalytic oxidation of gaseous ammonia: Stepping over nitrogen energy canyon. Angew. Chem. 136 (2024) e202404348. Gasdiffusionselektroden für die Elektrokatalytische Oxidation von gasförmigem Ammoniak: Das Überqueren des Thermodynamischen Tals der Stickstoffdreifachbindung 10.1002/anie.202404348
X. Wang, M. A. A. Mahbub, D. Das, W. Schuhmann, ChemCatChem 16 (2024) e202400601. Design of Bismuth-based electrocatalysts for carbon dioxide electroreduction 10.1002/cctc.202400601
R. Zerdoumi, A. Ludwig, W. Schuhmann, Curr. Opin. Electrochem. 48 (2024)101590. High entropy intermetallic compounds: A discovery platform for structure-property correlations and materials design principles in electrocatalysis 10.1016/j.coelec.2024.101590
L. Li, R. P. Antony, C. Santana Santos, N. Limani, S. Dieckhöfer, W. Schuhmann, Angew. Chem. Int. Ed. 63 (2024) e202406543. Anodic H2O2 generation in carbonate-based electrolytes – Mechanistic insight from scanning electrochemical microscopy. Angew. Chem. 136 (2024) e202406543. Anodische H2O2-Erzeugung in Elektrolyten auf Karbonatbasis – Mechanistische Erkenntnisse mittels elektrochemischer Rastermikroskopie 10.1002/anie.20240654 3
R. Zerdoumi, T. Quast, E. B. Tetteh, M. Kim, L. Li, S. Dieckhöfer, W. Schuhmann, Anal. Chem. 96 (2024) 10886-10892. Simultaneous mapping of activity and selectivity in electrocatalysis via a bifunctional nanopipette: Integration of scanning electrochemical microscopy and scanning electrochemical cell microscopy 10.1021/acs.analchem.4c00149
W. Costa Silva, M. Kim, G. Arruda de Oliveira, L. Vitor da Silva, E. B. Tetteh, C. Marina Rivaldo Gomez, W. Reis, B. Fragneaud, W. Schuhmann, C. Santana Santos, D. Grasseschi, Adv. Func. Mater. 34 (2024) 2403224. Investigation of doping effects on the local electrochemical activity of transition metal dichalcogenides 2D materials 10.1002/adfm.202403224
T. M. Benedetti, S. V. Sommerville, J. Wordsworth, Y. Yamamoto, W. Schuhmann, R. D. Tilley, J. J. Gooding, Adv. Func. Mater. 34 (2024) 2400322. An artificial enzyme: How nanoconfinement allows the selective electrochemical detection of glucose directly in whole blood 10.1002/adfm.202400322
L. Li, N. Limani, R. Antony, S. Dieckhöfer, C. Santana Santos, W. Schuhmann, Small Sci. 4 (2024) 2300283. Au micro- and nanoelectrodes as local voltammetric pH sensors during oxygen evolution at electrocatalyst-modified electrodes 10.1002/smsc.202300283
M. A. A. Mahbub, J. R. C. Junqueira, X. Wang, J. Zhang, S. Dieckhöfer, S. Seisel, D. Das, W. Schuhmann, Adv. Funct. Mater. 33 (2023) 2307752. Dynamic transformation of functionalized bismuth to multi-phase active sites for CO2 reduction to formate at high current densities 10.1002/adfm.202307752
E. Suhr, V. Strotkötter, O. A. Krysiak, W. Schuhmann, A. Ludwig, Adv. Eng. Mater. 25 (2023) 2300550. High-throughput exploration of structural and functional properties of the high entropy nitride system (Ti-Co-Mo-Ta-W)N 10.1002/adem.202300550
C. Møgelberg Clausen, O. Krysiak, L. Banko, J. K. Pedersen, W. Schuhmann, A. Ludwig, J. Rossmeisl, Angew. Chem. Int. Ed. 62 (2023) e202307187. A flexible theory for catalysis: Learning alkaline oxygen reduction on complex solid solutions within the Ag-Pd-Pt-Ru composition space.10.1002/anie.202307187
M. Kim, E. Batsa Tetteh, O. A. Krysiak, A. Savan, B. Xiao, T. Piotrowiak, C. Andronescu, A. Ludwig, T. D. Chung, W. Schuhmann, Angew. Chem. Int. Ed. 62 (2023) e202310069. Acidic hydrogen evolution electrocatalysis at high-entropy alloys correlates with its composition-dependent potential of zero charge 10.1002/anie.202310069
W. He, S. Chandra, T. Quast, S. Varhade, S. Dieckhöfer, J. R. C. Junqueira, H. Gao, S. Seisel, W. Schuhmann, Adv. Mater., Enhanced nitrate-to-ammonia efficiency over linear assemblies of copper-cobalt nanophases stabilized by redox polymers 10.1002/adma.202303050
C. Santana Santos, B. Nsolebna Jaato, I. Sanjuán, W. Schuhmann, C. Andronescu, Chem. Rev., 123 (2023) 4972-5019. Operando scanning electrochemical probe microscopy during electrocatalysis 10.1021/acs.chemrev.2c00766
J. R. C. Junqueira, D. Das, A. C. Brix, S. Dieckhöfer, J. Weidner, X. Wang, J. Shi, W. Schuhmann, ChemSusChem, Simultaneous anodic and cathodic formate production in a paired electrolyzer by CO2 reduction and glycerol oxidation 10.1002/cssc.202202349
P. Wilde, A. Özden, H. Winter, T. Quast, J. Weidner, S. Dieckhöfer, J. R. C. Junqueira, M. Metzner, W. Peter, W. Leske, D. Öhl, T. Bobrowski, T. Turek, W. Schuhmann, Appl. Res., Sprayed Ag gas-diffusion electrodes for the electrochemical reduction of CO2 to CO 10.1002/appl.202200081
V. Strotkötter, O. A. Krysiak, J. Zhang, X. Wang, E. Suhr, W. Schuhmann, A. Ludwig, Chem. Mater. Discovery of high-entropy oxide catalysts – From thin-film materials libraries to electrocatalyst particles 10.1021/acs.chemmater.2c01455
S. Somerville, P. O'Mara, T. M. Benedetti, S. Cheong, W. Schuhmann, R. Tilley, J. J. Gooding, J. Phys. Chem. C., Nanoconfinement allows a less active cascade catalyst to produce more C2+ products in electrochemical CO2 reduction 10.1021/acs.jpcc.2c07518
J. Zhang, W. He, T. Quast, J. R. C. Junqueira, S. Saddeler, S. Schulz, W. Schuhmann, Angew. Chem., Single-entity electrochemistry unveils tandem catalysis of Cu2O and Co3O4 for converting NO3− to NH3 10.1002/anie.202214830
L. Banko, E. Batsa Tetteh, A. Kostka, T. H. Piotrowiak, O. A. Krysiak, U. Hagemann, C. Andronescu, W. Schuhmann, A. Ludwig, Adv. Mater., Microscale combinatorial libraries for the discovery of high entropy materials 10.1002/adma.202207635
X. Wang, W. He, J. Shi, J. R. C. Junqueira, J. Zhang, S. Dieckhöfer, S. Seisel, D. Das, W. Schuhmann, Chem. Asian J., Ag-induced phase transition of Bi2O3 nanofibers for enhanced energy conversion efficiency towards formate in CO2 electroreduction 10.1002/asia.202201165
X. Wang, C. Tomon, T. Bobrowski, P. Wilde, J. R. C. Junqueira, T. Quast, W. He, N. Sikdar, J. Weidner, W. Schuhmann, ChemElectroChem 9 (2022) e202200675. Gaining the freedom of scalable gas diffusion electrodes for the CO2 reduction reaction 10.1002/celc.202200675 10.1002/celc.202200675
S. Schumacher, L. Madauß, Y. Liebsch, E. Batsa Tetteh, S. Varhade, W. Schuhmann, M. Schleberger, C. Andronescu, ChemElectroChem 9 (2022) e202200586. Revealing the heterogeneity of large-area MoS2 layers in the electrocatalytic hydrogen evolution reaction 10.1002/celc.202200586
O. A. Krysiak, S. Schumacher, A. Savan, W Schuhmann, A. Ludwig, C. Andronescu, Nano Res. 15 (2022) 4780−4784. Searching novel complex solid solution electrocatalysts in unconventional element combinations.10.1007/s12274-021-3637-z
E. Batsa Tetteh, L. Banko, O. A. Krysiak, T. Löffler, B. Xiao, S. Varhade, S. Schumacher, A. Savan, C. Andronescu, A. Ludwig, W. Schuhmann, Electrochem. Sci. Adv. 2 (2022) e2100105. Zooming-in – Visualisation of active site heterogeneity in high entropy alloy electrocatalysts using scanning electrochemical cell microscopy 10.1002/elsa.202100105
W. He, J. Zhang, S. Dieckhöfer, S. D. Varhade, A. C. Brix, A. Lielpetere, S. Seisel, J. R. C. Junqueira, W. Schuhmann, Nature Comm. 13 (2022) 1129. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. 10.1038/s41467-022-28728-4
J. Zhang, T. Quast, W. He, S. Dieckhöfer, J. R. C. Junqueira, D. Öhl, P. Wilde, D. Jambrec, Y.-T. Chen, W. Schuhmann, Adv. Mater. 34 (2022) 2109108. In-situ carbon corrosion and Cu leaching as a strategy for boosting oxygen evolution reaction in multi-metal electrocatalysts 10.1002/adma.202109108
N. Sikdar, J. R. C. Junqueira, D. Öhl, S. Dieckhöfer, T. Quast, M. Braun, H. B. Aiyappa, S. Seisel, C. Andronescu, W. Schuhmann, Chem. Eur. J. 28 (2022) e202104249. Redox replacement of Ag on MOF-derived Cu/C-nanoparticles on gas diffusion electrodes for electrocatalytic CO2 reduction 10.1002/chem.202104249
E. Batsa Tetteh, T. Löffler, T. Tarnev, T. Quast, P. Wilde, H. Barike Aiyappa, S. Schumacher, C Andronescu, R. D. Tilley, X. Chen, W. Schuhmann, Nano Res. 15 (2022) 1564-1569. Calibrating SECCM measurements by means of a nanoelectrode ruler. The intrinsic oxygen reduction activity of PtNi catalyst nanoparticles. 10.1007/s12274-021-3702-7
J. R. C. Junqueira, P. B. O’Mara, P. Wilde, T. M. Benedetti, C. Andronescu, R. D. Tilley, J. J. Gooding, W. Schuhmann, ChemElectroChem 8 (2021) e202100906. Combining nanoconfinement in Ag core/porous Cu shell nanoparticles with gas diffusion electrodes for improved electrocatalytic carbon dioxide reduction 10.1002/aenm.202102858
M. C. O. Monteiro, S. Dieckhöfer, T. Bobrowski, T. Quast, D. Pavesi, M. T. M. Koper, W. Schuhmann, Chem. Sci. 12 (2021) 15682-15690. Probing the local activity of CO2 reduction on gold gas diffusion electrodes: effect of the catalyst loading and CO2 pressure 10.1039/d1sc05519d
J. K. Pedersen, C. M. Clausen, O. A. Krysiak, B. Xiao, T. A. A. Batchelor, T. Löffler, V. A. Mints, L. Banko, M. Arenz, A. Savan, W. Schuhmann, A. Ludwig, J. Rossmeisl, Angew. Chem. Int. Ed. 60 (2021) 24144-24152. Bayesian optimization of high-entropy alloy compositions for electrocatalytic oxygen reduction 10.1002/anie.202108116
T. Quast, S. Varhade, S. Saddeler, Y.-T. Chen, C. Andronescu, S. Schulz, W. Schuhmann, Angew. Chem. Int. Ed. 60 (2021) 23444-23450. Single particle nanoelectrochemistry reveals the catalytic oxygen evolution reaction activity of Co3O4 nanocubes. Angew. Chem. 133 (2021) 23634-23640. Einzelpartikel-Nanoelektrochemie für die Untersuchung der Aktivität der elektrokatalytischen Sauerstoffentwicklungsreaktion an Co3O4 Nanowürfeln. 10.1002/anie.202109201
N. Sikdar, J. R. C. Junqueira, S. Dieckhöfer, T. Quast, M. Braun, Y. Song, H. B. Aiyappa, S. Seisel, J. Weidner, D. Öhl, C. Andronescu, W. Schuhmann, Angew. Chem. Int. Ed. 60 (2021) 23427-23434. A metal-organic framework derived CuxOyCz catalyst for electrochemical CO2 reduction and impact of local pH change. Angew. Chem. 133 (2021) 23616-23624. Ein MOF-basierter CuxOyCz-Katalysator für die elektrochemische CO2-Reduktion und die Auswirkungen der lokalen pH-Änderung 10.1002/anie.202108313
J. Zhang, W. He, H. Barike Aiyappa, T. Quast, S. Dieckhöfer, D. Öhl, Y.-T. Chen, J. Masa, W. Schuhmann, Adv. Func. Mater. 8 (2021) 2100041. Hollow CeO2@Co2N nanosheets derived from Co-ZIF-L for boosting the oxygen evolution reaction 10.1002/admi.202100041
Y. Song, J. R. C. Junqueira, N. Sikdar, D. Öhl, S. Dieckhöfer, T. Quast, S. Seisel, J. Masa, C. Andronescu, W. Schuhmann, Angew. Chem. Int. Ed. 60 (2021) 9135-9141. B-Cu-Zn gas diffusion electrodes for CO2 electroreduction to C2+ products at high current densities 10.1002/anie.202016898
P. Wilde, P. B. O’Mara, J. R. C. Junqueira, T. Tarnev, T. Benedetti, C. Andronescu, Y.-T. Chen, R. D. Tilley, W. Schuhmann, J. J. Gooding, Chem. Sci. 12 (2021) 4028-4033. Is Cu instability during the CO2 reduction reaction governed by the applied potential or the local CO concentration? 10.1039/d0sc05990k
S. Dieckhöfer, D. Öhl, J. R. C. Junquiera, T. Quast, T. Turek, W. Schuhmann, Chem. Eur. J. 27 (2021) 5906-5912. Probing the local reaction environment during high turnover carbon dioxide reduction with Ag-based gas diffusion electrodes 10.1002/chem.202100387
T. A. A. Batchelor, T. Löffler, B. Xiao, O. A. Krysiak, V. Strotkötter, J. K. Pedersen, C. M. Clausen, A. Savan, Y. Li, W. Schuhmann, J. Rossmeisl, A. Ludwig, Angew. Chem. Int. Ed., 60 (2021) 6932-6937. Complex solid solution electrocatalyst discovery by computational prediction and high-throughput experimentation 10.1002/anie.202014374
T. Quast, H. Barike Aiyappa, S. Saddeler, P. Wilde, Y.-T. Chen, S. Schulz, W. Schuhmann, Angew. Chem. Int. Ed. 60 (2021) 3576-3580. Single entity electrocatalysis of individual ‘picked-and-dropped’ Co3O4 nanoparticles on the tip of a carbon nanoelec-trode. Angew. Chem. 133 (2021) 3619-3624. Elektrokatalyse einzelner, auf der Spitze einer Kohlenstoff-Nanoelektrode platzierter Co3O4-Nanopartikel 10.1002/anie.202014384
Y. S. Lee, A. Ruff, R. Cai, K. Lim, W. Schuhmann, S. D. Minteer, Angew. Chem. Int. Ed. 59 (2020) 16511-16516. Electroenzymatic nitrogen fixation using an organic redox polymer-immobilized MoFe protein system. Angew. Chem. 132 (2020) 16654-16659. Elektroenzymatische Stickstofffixierung unter Verwendung eines MoFe-Proteinsystems immobilisiert in einem organischen Redoxpolymer 10.1002/anie.202007198
J. Masa, C. Andronescu, W. Schuhmann, Angew. Chem. Int. Ed. 59 (2020) 15298-15312. Electrocatalysis as the nexus for sustainable renewable energy. The Gordian knot of activity, stability, and selectivity 10.1002/anie.202007672
S. Möller, S. Barwe, S. Dieckhöfer, J. Masa, C Andronescu, W. Schuhmann, ChemElectroChem 7 (2020) 2680-2686. Differentiation between carbon corrosion and oxygen evolution catalyzed by NixB/C hybrid electrocatalysts in alkaline solution using differential electrochemical mass spectrometry 10.1002/celc.202000697
T. Tarnev, S. Cychy, C. Andronescu, M. Muhler, W. Schuhmann, Y.-T. Chen, Angew. Chem. Int. Ed. 59 (2020) 5586-5590. A universal nano-capillary based method of catalyst immobilization for liquid cell transmission electron microscopy. Angew. Chem. 132 (2020) 5634-5638. Eine universelle, auf Nanokapillaren basierende Methode zur Katalysatorimmobilisierung für die Flüssigzell-Transmissionselektronenmikroskopie 10.1002/anie.201916419
P. B. O’Mara, P. Wilde, T. M. Benedetti, C. Andronescu, S. Cheong, J. J Gooding, R. D. Tilley, W Schuhmann; J. Am. Chem. Soc. 141 (2019) 14093-14097. Cascade reactions in nanozymes: Spatial confinement in Ag-Cu bimetallic nanoparticles for carbon dioxide reduction 10.1021/jacs.9b07310
Room:
NC 04/788
Phone: +49 234 32 -
26200
E-Mail